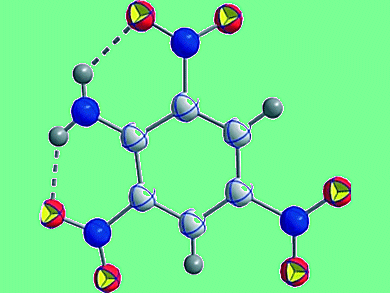
Formation of a chain of intramolecular hydrogen bonds around a central benzene ring can be competitive with steric hindrance of the substituents and can modify the aromaticity of the benzene ring.
Irena Majerz and Teresa Dziembowska, University of Wrocław, and Pomeranian University of Technology, Szczecin, Poland, show the influence of intramolecular hydrogen bonds and steric hindrance on distortion from planarity of the benzene ring and its aromaticity for a series of substituted 2,4,6-trinitroanilines.
They compare the crystal structure geometry and the geometry optimized at the B3LYP/6-311++G** level with analogues without intramolecular hydrogen bonds. The HOMA index and the parameter ΔP describing the distortion from planarity of the benzene ring were used to characterize the aromacity of the investigated molecules. NBO and AIM analysis were also applied.
- Geometric Aspects of Aromaticity: Interrelations between Intramolecular Hydrogen Bonds, Steric Effects and π-Electron Delocalisation in Nitroanilines,
Irena Majerz, Teresa Dziembowska
Eur. J. Org. Chem. 2010.
DOI: 10.1002/ejoc.201000978