Although marine macrolides Mandelalides A–D (variously glycosylated, unusual polyketide macrolides) show interesting anticancer activities, their availability is often problematic. Sub-mg quantities have been obtained from the new Lissoclinum species by using activity-guided fractionation, but these tiny amounts are often insufficient to be able to identify the drug’s biochemical and pharmacological effects.
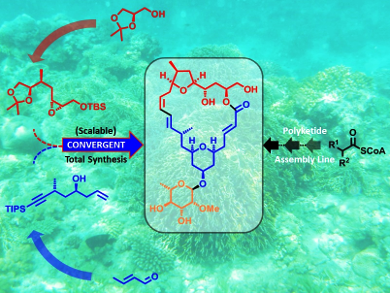
To provide a reliable synthetic supply of these marine macrolides, Karl-Heinz Altman and co-workers, Swiss Federal Institute of Technology (ETH), Zürich, have established a new total synthesis (comprising 18 steps overall for the longest linear sequence from the known ester), isolating Mandelalide A in an overall yield of 2.15 %. The final construction of the 24-membered macrolide core structure involved a high-yielding Sonogashira coupling of two complex fragments and a remarkably effective Shiina macrolactonization. The ultimate stereogenic step could be successfully performed on a multigram scale. Therefore, this work provides a reliable stock of natural Mandelalide A and also offers a platform for the synthesis of its analogues and tool compounds.
After biological evaluation on different human cancer cell lines, the team revealed a surprising behavior – Mandelalide A’s activity is highly cell-type-dependent and not a general cytotoxic compound as previously thought. A reliable synthetic source of this compound may accelerate investigations into the mechanism of action of Mandelalide A and identification of its molecular target(s), which could have interesting implications for future anticancer drug discovery.
- Total Synthesis and Biological Assessment of Mandelalide A,
Tobias Michael Brütsch, Pascal Bucher, Karl-Heinz Altmann,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201504230