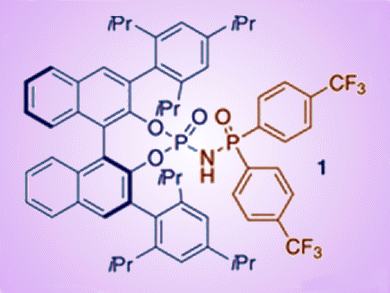
Researchers led by Benjamin List, Max Planck Institute in Mülheim, Germany, have reported the direct enantioselective synthesis of cyclic aminal compounds from aldehydes through a sequence consisting of imine formation and intramolecular amidation. To do this, they designed a novel chiral Brønsted acid catalyst by introducing a phosphinyl amido moiety (see catalyst 1). With bringing in new substituents, these catalysts were easily modified and fine-tuned for a particular reaction (see scheme).
List’s methodology was used in the first catalytic asymmetric synthesis of the pain killer (R)-chlorothenoxazine. It is envisioned that the N,O-acetals prepared by this approach will be useful intermediates for the synthesis of biologically active compounds.

- N-Phosphinyl Phosphoramide—A Chiral Brønsted Acid Motif for the Direct Asymmetric N,O-Acetalization of Aldehydes
S. Vellalath, I. Čoric´, B. List,
Angew. Chem. Int Ed. 2010, 49.
http://dx.doi.org/10.1002/anie.201005347 - S. Vellalath, I. Čoric´, B. List,
Angew. Chem. 2010, 122.
http://dx.doi.org/10.1002/ange.201005347