Preparing Transfer RNA
Ribonucleic acid (RNA) is a biologically important molecule that is very similar to DNA, the blueprint of life. Naturally occurring RNAs, such as transfer RNA (tRNA), contain modified building blocks (“nucleosides”), which are involved in decoding genetic information. Deazaguanosine nucleosides, in particular, are of significant interest for their antibacterial, antifungal, antiviral, and anticancer activity. Thomas Carell and his team at Munich’s Ludwig Maximilians University, Germany, have introduced a method to prepare tRNA nucleosides through a novel Turbo-Grignard-based approach with an unprecedented level of control from a common intermediate.
Because of the biological importance of deazaguanosines, a reliable method for their preparation is desirable. In this way, scientists can easily study their functions and the role they play in the treatment of diseases. One of the problems for synthetic chemists, however, is that these compounds often contain various reactive groups at several locations within the molecule. Precise control over the reactivity at a single position can therefore be difficult. Thus, the development of a site-specific reagent is required.
Site-Specific Turbo-Grignard
For their synthesis, the researchers opted to use the versatile Turbo-Grignard reagent. The “normal” Grignard reagent is used by chemists to introduce a group into a molecule at a reactive site; it consists of the group to be added complexed to the metal magnesium. The Turbo-Grignard is also complexed to a lithium salt, which generally allows reactions to be performed under mild conditions – an important advantage when dealing with biologically relevant compounds.
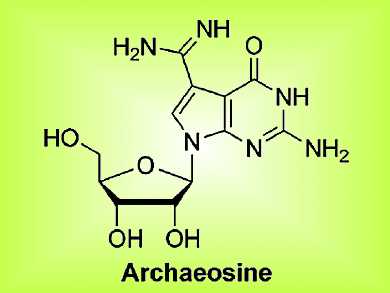
In their article, the team shows that the Turbo-Grignard reagent has a specific point of attack and that it can be used in the presence of other reactive groups. Importantly, an adjacent group that proved problematic under different conditions was found to be completely unreactive to the turbo reagent, thereby allowing efficient synthesis of the desired nucleosides; the same reaction performed with the “normal” Grignard reagent resulted in decomposition of the desired products. The team used this methodology to synthesize the deazaguanosine-derived natural products PreQ1, PreQ0, and archaeosine (see figure).
The fact that other reactive groups in the molecule remain untouched facilitates the synthesis of deazaguanosine-derived tRNA nucleosides, which should enable detailed biochemical investigation of their functions in vivo and help in the treatment of genetic diseases.
- Efficient Synthesis of Deazaguanosine-Derived tRNA Nucleosides PreQ0, PreQ1, and Archaeosine Using the Turbo-Grignard Method
T. Brückl, I. Thoma, A. J. Wagner, P. Knochel, T. Carell
Eur. J. Org. Chem. 2010.
DOI: 10.1002/ejoc.201000987