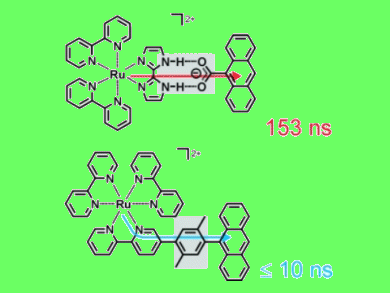
Energy-transfer reactions play a key role in the antenna system of natural photosynthesis, and a thorough understanding of all factors that govern energy transfer rates is important for the development of artificial light harvesting systems. In this context, two artificial model systems comprising a ruthenium complex and an anthracene moiety were synthesized by Jonathan Freys and Oliver Wenger, Georg-August-University, Germany, and investigated by spectroscopic methods.
When excited by short laser pulses, the ruthenium complex donates the absorbed light energy to the anthracene acceptor. This occurs with rates that are governed by the linker connecting the ruthenium donor and the anthracene acceptor: In the case of a salt bridge, energy transfer is associated with a time constant of 153 ns, while for a covalent (xylene) linker it occurs within less than 10 ns.
This direct comparison of energy transfer across a salt bridge and a covalent linker is helpful for elucidating the pathways of energy flow in proteins that involve these two types of linkages between their donor and acceptor sites.
- Supramolecular and Intramolecular Energy Transfer with Ruthenium–Anthracene Donor–Acceptor Couples: Salt Bridge versus Covalent Bond
J. C. Freys, O. S. Wenger,
Eur. J. Inorg. Chem. 2010.
DOI: 10.1002/ejic.201000815