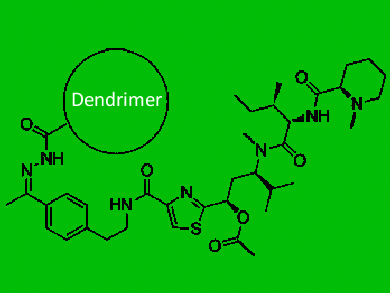
Tubulysins are a family of natural products with interesting structural properties and exceedingly potent cytotoxicity, which is brought about by their inhibition of tubulin polymerization and subsequent induction of apoptosis. They have, therefore, been the subject of research efforts in synthesis, biosynthesis, structure–activity relationships, bioactivity, mode of action, and chemotherapeutic potential.
A collaborative team led by Francis Szoka, University of California, San Francisco, Jean Fréchet, University of California, Berkeley, and John Ellman, Yale University, USA, report the first application of polymeric drug delivery using synthetic tubulysin analogues, which overcome many of the liabilities of the naturally occurring tubulysins, including ease of synthesis and high stability. The application of this dendrimer conjugation technology has resulted in promising efficacy without toxicity in animal trials.
- Chemotherapeutic Evaluation of a Synthetic Tubulysin Analogue–Dendrimer Conjugate in C26 Tumor Bearing Mice
W. C. Floyd III, G. K. Datta, S. Imamura, H. M. Kieler-Ferguson, K. Jerger et al.,
ChemMedChem 2011, 6 (01).
DOI: 10.1002/cmdc.201000377