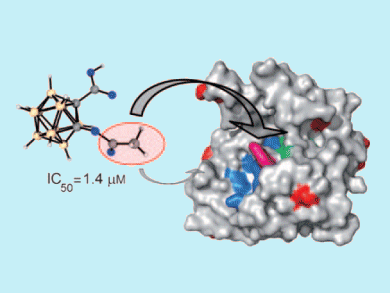
Aspirin exerts its anti-inflammatory effects through inhibition of cyclooxygenase (COX) enzymes by acetylating a specific active site serine residue. Because aspirin is such a well-characterized drug, Evamarie Hey-Hawkins and colleagues, University of Leipzig, Germany, used it as a template for their research into ortho-carbaborane derivatives.
Hey-Hawkins’ team found that asborin, the carbaborane-containing analogue of aspirin, also inhibits COX enzymes, but more weakly and through a different mechanism of action: by acetylating surface-exposed lysine residues. Asborin was found to be a potent inhibitor of aldo/keto reductase (AKR) 1A1, and lysine acetylation was found to be crucial for this.
AKR1A1 is one of a large family of reductases, and other members have been shown to play important roles in a variety of cancers and neurological disorders. Asborin holds promise as a lead structure for AKR inhibition because the carbon/boron cluster can be fine-tuned for other AKR targets.
- Asborin Inhibits Aldo/Keto Reductase 1A1
M. Scholz, M. Steinhagen, J. T. Heiker, A. G. Beck-Sickinger, E. Hey-Hawkins,
ChemMedChem 2011, 6 (01).
DOI: 10.1002/cmdc.201000368