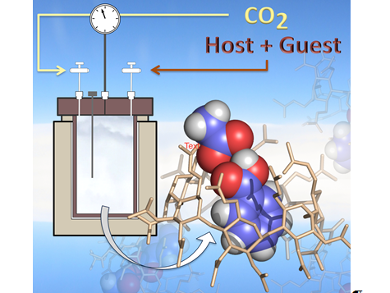
Supercritical carbon dioxide (scCO2) is a “green” alternative to traditional organic solvents, but polar molecules and high molar mass compounds are often poorly soluble in it. However, a clear molecular picture of the reasons preventing, or allowing, inclusion complexes to be formed in scCO2 is yet to be realized.
Francesca Ingrosso, Alain Marsura, Manuel F. Ruiz-López, and co-workers, Université de Lorraine, Vandœuvre-lès-Nancy, France, have designed the first stable inclusion complex formed in scCO2 between a guest organic molecule (a polar aromatic molecule (e.g., benzoic acid)) and an scCO2-soluble host, namely, peracetylated β-cyclodextrin (per-β-CD).
The team characterized the stable inclusion complex in scCO2 by using TGA, X-ray diffraction, solid state CP/MAS NMR spectroscopy, as well as by Molecular Dynamic simulations. Further analysis of the molecular dynamics trajectories enabled the group to analyze the nature of the driving force for the formation of the complex. The team found that a polar guest with hydrogen-bond donor capacity is able to force the opening of the cavity and form the stable inclusion complex.
These results may lead the way to green applications of scCO2 in many areas of chemistry, including supramolecular synthesis, reactivity and catalysis, micro- and nanoparticle formation, molecular recognition, as well as enhanced extraction processes with increased selectivity.
- Driving Forces Controlling Host–Guest Recognition in Supercritical Carbon Dioxide Solvent,
Francesca Ingrosso, Muhannad Altarsha, Florence Dumarçay, Gwendal Kevern, Danielle Barth, Alain Marsura, Manuel F. Ruiz-López,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201503780