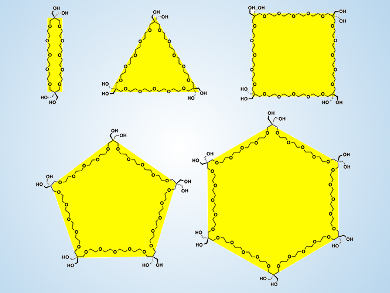
Poly(ethylene glycol) (PEG) is a neutral, linear, functional polyether that is both water-soluble and biocompatible. Fine-tuning its thermal response is of interest since changing its characteristics could allow the molecular design of functional nonionic surfactants and macromolecules with stimuli-responsive self-assembling properties.
Kazushi Kinbara, Tokyo Institute of Technology, Yokohama, Japan, and colleagues have synthesized new monodisperse polygonal PEG analogues from digonal to hexagonal structures. The team achieved this by connecting ethylene glycol oligomers with acetal-protected pentaerythritol units that introduce an extra carbon-atom “vertex” into the polyether chain.
All the polygonal PEGs are water-miscible at room temperature, but ring expansion monotonically enhances the hydrophobicity. Upon heating, only trigonal and hexagonal PEGs form aggregates in water, while tetragonal and pentagonal PEGs hardly respond to heating. These specific thermal responses are attributed to an effect by which the topology of the polygonal PEGs, rather than their size, influences both intramolecular packing and local dynamics of molecules, which further affects the thermoresponsive self-assembly in aqueous media.
- Synthesis and Thermal Responses of Polygonal Poly(ethylene glycol) Analogues,
Shunichi Kawasaki, Takahiro Muraoka, Tsutomu Hamada, Kazuki Shigyou, Fumi Nagatsugi, Kazushi Kinbara,
Chem. Asian J. 2016.
DOI: 10.1002/asia.201501381