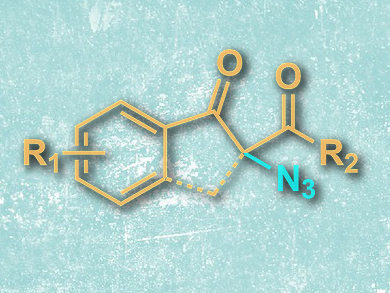
The synthesis of azides is a way to incorporate nitrogen into organic compounds, as well as a method to generate reactants for click chemistry. The azidation of 1,3-dicarbonyl compounds, however, is problematic, as both the substrate and the azide are nucleophilic. Hypervalent iodine reagents can overcome this hurdle, but are usually employed in stoichiometric amounts.
Ramaiah Prabhu and colleagues, Indian Institute of Science, Bangalore, India, have developed a synthesis of tertiary azides from 1,3-dicarbonyl compounds that employs the iodine reagent tetrabutylammonium iodide (TBAI) in catalytic amounts only. In addition to TBAI, tert-butyl hydroperoxide (TBHP) is used as an oxidant.
The mechanism proposed for the reaction includes the in situ generation of hypoiodite from TBAI and TBHP, which reacts with the carbonyl compound. This intermediate is then azidated and the hypoiodite regenerated by oxidation. The mild and “green” reaction proceeds in water, and allows the synthesis of a variety of azide-functionalized β-keto esters, amides, and ketones in good yields.
- An Efficient Tertiary Azidation of 1,3-Dicarbonyl Compounds in Water Catalyzed by Tetrabutylammonium Iodide,
Jayaraman Dhineshkumar, Kandikere Ramaiah Prabhu,
Eur. J. Org. Chem. 2015.
DOI: 10.1002/ejoc.201501374