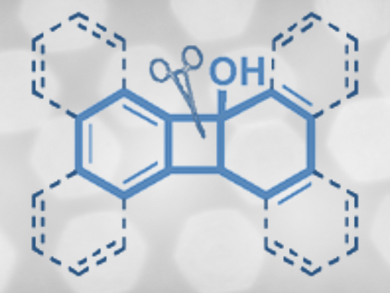
Polycyclic aromatic hydrocarbons (PAHs) are a class of compounds with a wide range of possible applications, e.g., as organic materials or as a framework for pharmaceutically active molecules. Current syntheses usually employ the intramolecular cyclization of complex precursors, which limits the product scope.
Chen Zhu and colleagues, Soochow University, Suzhou, China, have developed an iridium-catalyzed PAH synthesis that involves an intermolecular cyclization between ring-fused benzocyclobutenols (RBCBs, pictured) and alkynes. The RBCBs can be easily synthesized from commercially available arylbromides and ketones. The iridium complex [{Ir(OMe)(COD)2}2] (COD = 1,5-cyclooctadiene) was used as the catalyst. During the reaction, one of the C–C bonds in the cyclobutane subunit is cleaved and the alkyne is inserted.
The reactions proceed in good to excellent yields under mild conditions, and tolerate a range of functional groups. The approach allows the facile synthesis of a variety of PAHs with multiple subtituents.
- Synthesis of Multiply Substituted Polycyclic Aromatic Hydrocarbons by Iridium-Catalyzed Annulation of Ring-Fused Benzocyclobutenol with Alkyne through C–C Bond Cleavage,
Jiajia Yu, Hong Yan, Chen Zhu,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201509973