The sluggish kinetics of the oxygen reduction reaction (ORR) at the cathodes of fuel cells significantly hampers fuel cell performance. Therefore, the development of high-performance, non-precious-metal catalysts as alternatives to platinum-based ORR electrocatalysts is highly desirable for the large-scale commercialization of fuel cells.
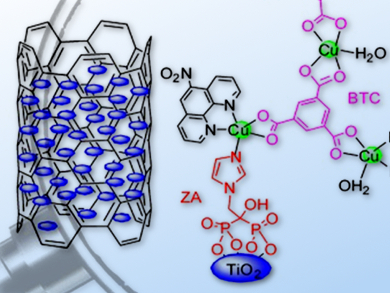
Jin-Gang Liu and colleagues, East China University of Science and Technology, Shanghai, China, have developed TiO2-grafted copper complexes deposited on multiwalled carbon nanotubes (CNTs), which form stable and efficient electrocatalysts for the ORR. The optimized catalyst composite shows surprisingly high selectivity for the four-electron reduction of O2 to water (approximately 97 %) in alkaline solution, and demonstrates superior stability and excellent tolerance for the methanol crossover effect in comparison to a commercial Pt/C catalyst.
This facile approach to the assembly of copper catalysts on TiO2 with rationally tuned ORR activity could have significant implications for the development of high-performance, non-precious-metal ORR catalysts.
- Titanium Dioxide-Grafted Copper Complexes: High-Performance Electrocatalysts for the Oxygen Reduction Reaction in Alkaline Media,
Fei-Fei Wang, Ping-Jie Wei, Guo-Qiang Yu, Jin-Gang Liu,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201502589