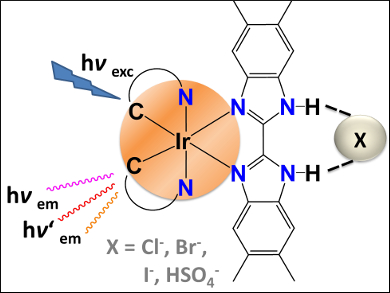
Anion sensors that function under ambient conditions are important for the detection of anions that play fundamental roles in a range of chemical, biological, and environmental processes. A practical method is to record the quenching or enhancement of a spectroscopic signal upon interaction of the sensor with the species to be detected.
Sven Rau and colleagues, Ulm University, Germany, studied the changes in the emission, NMR, and IR absorption spectra of the iridium(III)–bibenzimidazole complex [Ir(ppy)2(tmBBI-H2)](PF6) (IrBBI-H2) (ppy = phenylpyridine) upon its interaction with various anions such as halides, acetate, H2PO4–, and HSO4–. They found that the response generated by the anions could be detected under ambient conditions by simple methods such as NMR titration and emission spectroscopy.
The team showed that the photophysics of the complex was influenced by hydrogen bonds in the ligand periphery. The complex is a convenient sensor for various anions under ambient conditions.
- Interaction of an Iridium(III)-Bibenzimidazole Complex with Anions – Implications for Luminescent Sensing,
Sebastian A. Rommel, Dieter Sorsche, Apoorva Dixit, Sven Rau,
Eur. J. Inorg. Chem. 2015.
DOI: 10.1002/ejic.201501108