Imaging Probes
Radioactively labeled compounds are important for use as imaging probes or radiotherapeutic agents. Most radioisotopes with suitable decay properties are transition metals or elements with metallic character. Therefore, bifunctional chelators are required for stable tethering of a given radionuclide to targeted biomolecules. However, preparing such multifunctional radioconjugates by conventional synthetic means is a significant challenge. To address this, a collaboration led by Thomas Mindt at University Hospital Basel and the University of Basel in Switzerland applied click chemistry toward the efficient assembly of conjugates containing a 99mTc-based single photon emission computed tomography (SPECT) probe and two different biochemical entities, and their work is reported in ChemMedChem.
Selective Combination
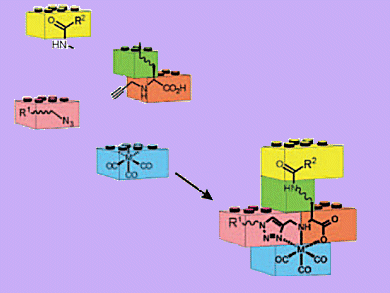
Starting from azide-functionalized biomolecules and readily available alkyne pro-chelators derived from amino acids, Mindt and colleagues assembled multifunctional conjugates by convenient one-pot procedures without the need for protecting groups. They applied this copper(I)-catalyzed alkyne–azide cycloaddition (CuAAC) methodology to the preparation of a 99mTc-labeled conjugate comprising a tumor-targeting peptide sequence (bombesin(7–14)) and a low-molecular-weight albumin binder (a pharmacological modifier that prolongs the conjugate’s blood circulation lifetime).
The possibility of selectively combining two different groups with biological function and a metal chelating system in a single step is a very attractive feature for the development of metallic radiotracers and opens numerous applications with great potential, including the design of radiopharmaceuticals with improved biological characteristics, multimodality imaging probes, bivalent or bi-specific targeting agents, and conjugates useful for combination therapies.
- Molecular Assembly of Multifunctional 99mTc Radiopharmaceuticals Using “Clickable” Amino Acid Derivatives
T. Mindt, H. Struthers, B. Spingler, L. Brans, D. Tourwé, E. García-Garayoa, R. Schibli,
ChemMedChem 2010, 5.
DOI: 10.1002/cmdc.201000342