The mechanistic differences between tri- and tetramerization of ethane have recently been gaining interest in order to find new catalytic systems.
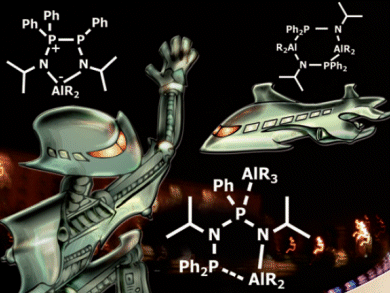
Uwe Rosenthal and co-workers, Rostock University, Germany, have studied the coordination, deprotonation, rearrangement, and cleavage of the ethene trimerization ligand Ph2PN(iPr)P(Ph)N(iPr)H by trialkylaluminium compounds.
With increasing temperature and residence time the reaction sequence from Ph2PN(iPr)P(Ph)N(iPr)H via the adduct [Ph2PN(iPr)P(Ph)N(iPr)H][AlR3], the aluminium amide [Ph2PN(iPr)P(Ph)(AlR3)N(iPr)][AlR2], the rearranged cyclic compound [N(iPr)P(Ph)P(Ph2)N(iPr)][AlR2], and finally the cleaved cyclic dimer [R2AlN(iPr)P(Ph)2]2 is observed.
The system consists of the trimerization ligand, CrCl3(THF)3, and Et3Al; this produces 1-hexene in more than 90 % yield with high purity and provides vital information for activation and deactivation mechanisms.
- Activation and Deactivation by Temperature: Behavior of Ph2PN(iPr)P(Ph)N(iPr)H in the Presence of Alkylaluminum Compounds Relevant to Catalytic Selective Ethene Trimerization
S. Peitz, N. Peulecke, B. R. Aluri, B. H. Müller, A. Spannenberg et al.,
Chem. Eur. J. 2010, 16(40).
DOI: 10.1002/chem.201001936