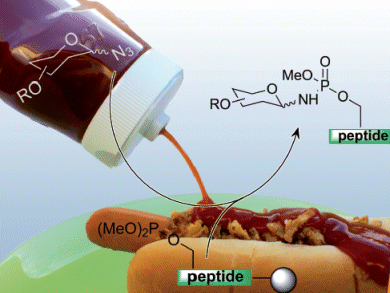
Glycoconjugate synthesis can provide artificial glycoconjugates, glycopeptide mimetics and naturally occurring glycopeptides and glycoproteins that are difficult to isolate from natural sources.
Christian Hackenberger and colleagues, Freie Universität Berlin, Germany, report a synthetic route to phosphoramidate-linked glycopeptides utilizing a solid-phase Staudinger phosphate reaction.
The key steps were a global phosphitylation of the unprotected Ser residue to a dimethylphosphite-containing peptide, glycosylation with a glycosyl azide, deprotection and cleavage from the resin of the phosphoramidate-linked glycopeptide by TFA treatment.
The glycopeptide mimetic formed contained a phosphoramidate linkage that was stable under acidic and physiological conditions.
- Solid-Phase Synthesis of Phosphoramidate-Linked Glycopeptides
D. M. M. Jaradat, H. Hamouda, C. P. R. Hackenberger,
Eur. J. Org. Chem. 2010, (26), 5004–5009.
DOI: 10.1002/ejoc.201000627