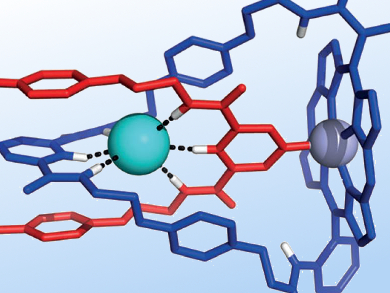
Templating effects have been exploited by chemists to create numerous intricate molecular architectures. While metal coordination remains the most widely employed class of templating interaction, anions have also been shown to act as templates, facilitating the interweaving of macrocycles and threads for the synthesis of catenanes.
Strapped-porphyrin-[2]catenanes synthesized in this manner by Paul Beer, University of Oxford, UK, and colleagues have been shown to demonstrate anion recognition and sensing properties. By using a zinc porphyrin macrocycle and a chloride template, the researchers were able to exploit anion templation, as well as pyridine-zinc ligation. They obtained the [2]catenane in 30 % yield through an amide-condensation-based clipping reaction.
1H NMR titration experiments on the PF6 salt of the catenane showed that it selectively binds chloride in the interlocked cavity, whereas neither bromide nor iodide showed any binding interaction. The catenane was also shown to exhibit modest optical chloride sensing capabilities using UV/Vis and fluorescence spectroscopy.
- Chloride-Anion-Templated Synthesis of a Strapped-Porphyrin-Containing Catenane Host System,
Asha Brown, Matthew J. Langton, Nathan L. Kilah, Amber L. Thompson, Paul D. Beer,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201502721