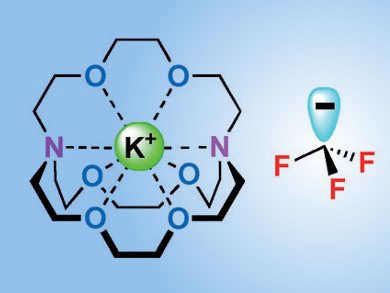
The trifluoromethyl group can make organic compounds more stable and more lipophilic, and is thus found in numerous pharmaceuticals and agrochemicals. The trifluoromethyl anion, CF3–, has long remained the putative intermediate in nucleophilic trifluoromethylation, a widely used transformation for the addition of this functional group. However, it was not until last year that chemists first confirmed its existence in the [K(18-crown-6)][CF3] ion pair [1].
Noting that the CF3– is still covalently bonded to the potassium cation in this previous study, Vladimir V. Grushin and colleagues, Institute of Chemical Research of Catalonia, Tarragona, Spain, switched the counter ion from [K(18-crown-6)] to [K(crypt-222)]+ and generated the free and “naked” CF3– in both solution and the solid state. Crypt-222 is the three-dimensional cage-like counterpart of the two dimensional crown ether, which not only coordinatively saturates the K+ cation, but sterically distances the CF3– anion.
The successful preparation of the truly free CF3– anion will on one hand advance the understanding of its basic physical organic chemistry, and might inspire the development of new trifluoromethylation reagents on the other.
- The Trifluoromethyl Anion,
Anton Lishchynskyi, Fedor M. Miloserdov, Eddy Martin, Jordi Benet-Buchholz, Eduardo C. Escudero-Adán, Andrey I. Konovalov, Vladimir V. Grushin,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201507356
References
- [1] Long-Lived Trifluoromethanide Anion: A Key Intermediate in Nucleophilic Trifluoromethylations,
G. K. Surya Prakash, Fang Wang, Zhe Zhang, Ralf Haiges, Martin Rahm, Karl O. Christe, Thomas Mathew, George A. Olah,
Angew. Chem. Int. Ed. 2014, 53, 11575–11578.
DOI: 10.1002/anie.201406505