Radioactive metal ions are useful in radiopharmaceuticals, i.e., drugs containing radioactive isotopes that can be used as diagnostic or therapeutic agents. The isotope 45Ti, for example, can be produced in large quantities using small commercial cyclotrons and could be useful in medical applications. However, Ti(IV) tends to form hydroxide species in aqueous solutions, which can be dehydrated to insoluble TiO2. Thus, Ti(IV) needs to be stabilized in coordination complexes.
Eszter Boros, Stony Brook University, New York, USA, and colleagues have developed a fragment-based screening approach to identify appropriate chelating ligands for Ti4+. Various bidentate chelators were screened for their ability to form 1:3 complexes with Ti4+ over a wide pH range. Suitable candidates were then used to generate hexadentate chelators with high complex stability in 1:1 complexes with Ti4+. The complexes were assessed for possible applications, e.g., in positron emission tomography (PET) imaging.

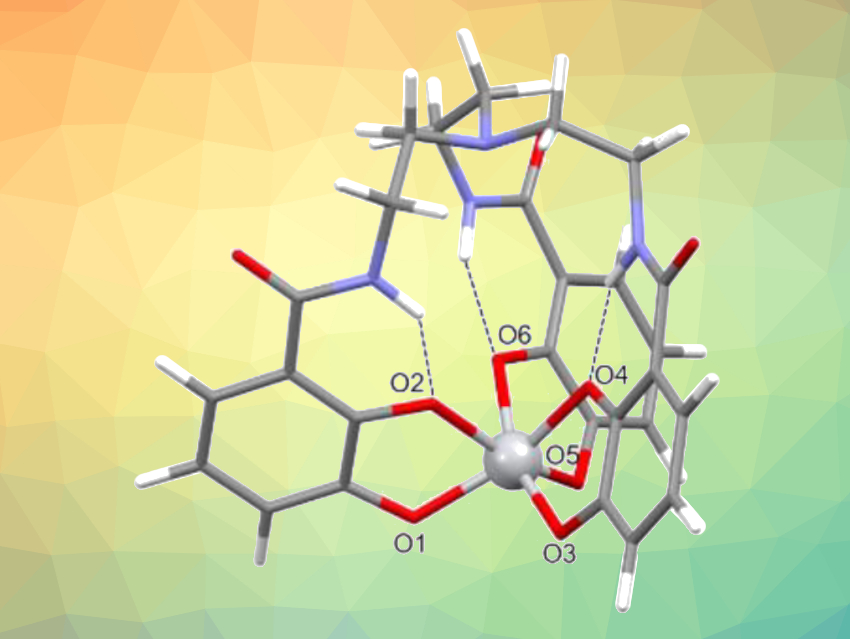
A chelating ligand made from catechol (benzene-1,2-diol) units, called TREN-CAM (pictured), captured and retained the 45Ti isotope with remarkable stability in vivo in mice (complex pictured at the top). Bifunctional versions of this chelator might allow the targeting of 45Ti to tissues of interest, opening up possibilities for the development of diagnostic radiopharmaceuticals.
- A General Design Strategy Enabling the Synthesis of Hydrolysis‐Resistant, Water‐Stable Titanium(IV) Complexes,
Angus J. Koller, Shefali Saini, Ivis F. Chaple, M. Andrey Joaqui‐Joaqui, Brett M. Paterson, Michelle T. Ma, Philip J. Blower, Valérie C. Pierre, Jerome R. Robinson, Suzanne E. Lapi, Eszter Boros,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202201211