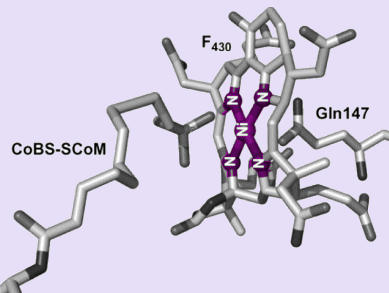
Methyl coenzyme M reductase (MCR) is a nickel enzyme that is necessary for methanogenesis, the biological formation of methane – the final step in the decay of biomass. These enzymes are found in methanogenic Archaea, which are single-celled microorganisms. In order to better understand the structure–function relationships of the more complex enzymes, it is important to find simpler compounds that can serve as models.
Kazuyuki Tatsumi et al. from Nagoya University, Japan, have synthesized a series of tetraazamacrocyclic nickel(II) complexes coordinated to methyl coenzyme M (MeSCoM) and coenzyme M (HSCoM), as models for the active site of MCR. Through X-ray crystallography, they determined that, in these complexes, MeSCoM and HSCoM are coordinated to the nickel centre at the axial position through the sulfonate O atom. Furthermore, the nickel complexes are octahedral, and the pendant pyridine-based ligand occupies the position trans to the sulfonate group.
These complexes closely resemble the active site of the MCRsilent form.
- Coordination of Coenzyme M and Its Derivatives on NiII(tetraazacycle) Complexes: A Model for the Active Site of Methyl Coenzyme M Reductase
J.-I. Nishigaki, T. Matsumoto, K. Tatsumi,
Eur. J. Inorg. Chem. 2010.
DOI: 10.1002/ejic.201000801