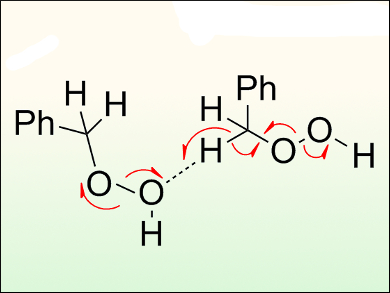
Hendrik Zipse and Lakshmanan Sandhiya, Ludwig Maximilian University, Munich, Germany, have investigated the thermochemical profiles for various radical-generating reactions involved in the (aut)oxidation of toluene using a computational analysis.
It appears that these reactions are unlikely to be initiated by triplet oxygen or the unimolecular decomposition of hydroperoxides. Instead, the bimolecular reaction between the hydroperoxides and toluene or a second equivalent of hydroperoxide is shown to be more favorable. This interpretation follows and supports the “molecule-induced homolysis” mechanism reported in the literature. The enthalpic driving force of this mechanism is the formation of a molecule of water together with two radicals.
The researchers compared several computational methods for their ability to describe the (aut)oxidation of hydrocarbons. The CBS-QB3 compound method performs best in reproducing the thermochemical data in the hydrogen atom transfer reactions.
- Initiation Chemistries in Hydrocarbon (Aut)Oxidation,
Lakshmanan Sandhiya, Hendrik Zipse,
Chem. Eur. J. 2015, 21, 14060–14067.
DOI: 10.1002/chem.201502384