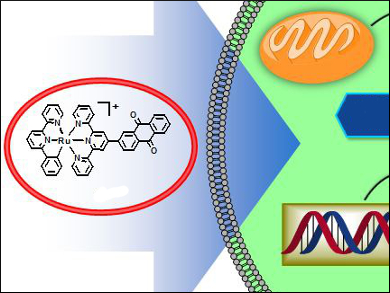
Drug resistance in solid tumors is a major problem in cancer treatment and drug discovery. Hypoxia in solid tumors plays a key role in cellular adaptation and the modulation of gene expression that leads to resistance to chemotherapy and radiotherapy.
Hui Chao and colleagues, Sun Yat-Sen University, Guangzhou, China, have demonstrated that the integration of cyclometalated ruthenium(II) complexes with anthraquinone ligands produces compounds that are highly toxic to hypoxic cancer cells.
The most effective complex was found to be [Ru(pbpy)(adtpy)](ClO4), (pbpy = 6-phenyl-2,2′-bipyridine; adtpy = 2-([2,2′:6′,2”-terpyridin]-4′-yl)anthracene-9,10-dione). The compound accumulated well in HeLa cancer cells, and exhibited 46-fold and 61-fold higher cytotoxic potency than cisplatin in hypoxic cells and 3D multicellular tumor spheroids.
Further studies demonstrated that this complex preferentially accumulated in the mitochondria of hypoxic HeLa cells, and induced apoptosis through multiple synergistic pathways. The complex inhibited DNA replication, promoted DNA damage and mitochondrial dysfunction, and inhibited HIF-1α in hypoxic HeLa cells, indicating that the complex is highly toxic to hypoxic tumor cells.
- Cyclometalated Ruthenium(II) Anthraquinone Complexes Exhibit Strong Anticancer Activity in Hypoxic Tumor Cells,
Leli Zeng, Yu Chen, Huaiyi Huang, Jinquan Wang, Donglei Zhao, Liangnian Ji, Hui Chao,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201502154