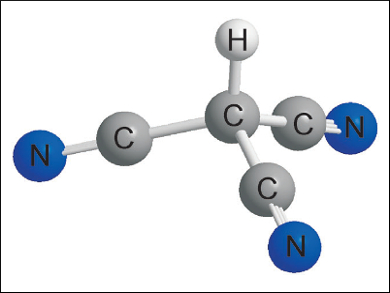
Tricyanomethane (cyanoform) is one of the strongest organic acids. Until now, it had only been identified by microwave spectroscopy in the gas phase at very low pressures, and attempts to isolate the compound had failed, or lead to its tautomer dicyanoketenimine.
Andreas J. Kornath and colleagues, Ludwig Maximilian University, Munich, Germany, have synthesized tricynomethane by a simple reaction of of calcium tricyanomethanide with anhydrous hydrogen fluoride. The colorless compound was found to be stable up to –40°C, and was characterized by 1H NMR, 13C NMR, 14N NMR, and Raman spectroscopy.
The researchers attribute the success of their synthesis mainly to the low reaction temperature of up to –50°C and the choice of hydrogen fluoride as both acid and solvent. The separation of the cyanoform from the by-product Ca(HF2)2 was found to be difficult and has not yet been achieved.
- The Existence of Tricyanomethane,
Theresa Soltner, Jonas Häusler, Andreas J. Kornath,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201506753