Oligoarginine sequences are well-know as cell-penetrating peptides that can be conjugated to other molecules to increase cell permeability. Adding oligoarginine sequences to proteins or peptides is, however, problematic because the products often have to be purified by HPLC and the “sticky” nature of oligoarginines renders this process somewhat inefficient.
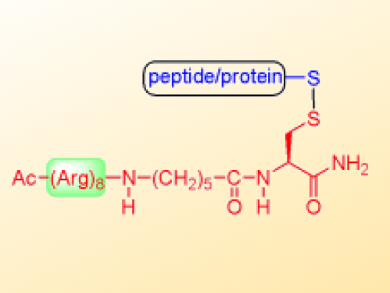
To combat this problem, Yoshi Hayashi and co-workers, Tokyo University of Pharmacy and Life Sciences, Japan, developed a thiol-selective, solid-supported, octaarginine-conjugating reagent, KSH-2, for the purpose of conjugating an octaarginine peptide to molecules with low membrane permeability to promote their intracellular delivery. This solid-supported reagent is prepared in two steps, including the activation of a 3-nitro-2-pyridinesulfenyl (Npys)-type resin by chlorosulfenylation and the loading of a Cys(t-Bu)-containing octaarginine unit to form an active disulfide.
In model experiments, KSH-2 efficiently introduced octaarginine peptides onto thiol-containing captopril and a hexadecapeptide via disulfide bonds and yielded the conjugated products with a high degree of purity by only mixing and filtration. KSH-2 could be a valuable reagent as it can be used to easily prepare octaarginine conjugates from a variety of thiol-containing compounds.
- Development of a thiol selective solid-supported oligoarginine-conjugating reagent KSH-2,
Kentarou Fukumoto, Akihiro Kajiyama, Shunsuke Shimura, Koji Taketa, Shinichiro Kimura, Akihiro Taguchi, Kentaro Takayama, Fumika Yakushiji, Yoshio Hayashi,
Asian J. Org. Chem. 2015.
DOI: 10.1002/ajoc.201500267