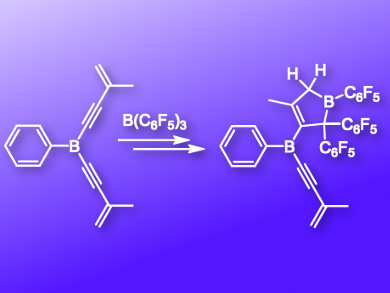
The electrocyclic pentadienyl to cyclopentenyl cation ring-closure reaction is an important synthetic transformation. The acid-induced Nazarov cyclization of divinylketones to cyclopentenones is an important variant. 1-Borylbutadienes are the neutral analogues of the pentadienyl cations, but their electrocyclic ring-closure is thermodynamically unfavorable unless it is accompanied by the subsequent migration of a substituent from boron to an adjacent carbon atom.
Gerhard Erker, Hellmut Eckert and colleagues, Universität Münster, Germany, have found an interesting new entry into such a bora-Nazarov reaction, namely by carrying out a 1,1-carboboration reaction (Wrackmeyer reaction) of a suitably substituted enyne with the strong boron Lewis acid B(C6F5)3. This generates a borylbutadiene system with the correct stereochemistry for a rapidly proceeding thermally induced bora-Nazarov cyclization, followed by a 1,2-C6F5 substituent migration to give the respective dihydroborole product (pictured).
1,1-Carboboration is currently used for the synthesis of alkenyl boranes, but also for substituted siloles, phospholes and even boroles.
- A 1,1-Carboboration Route to Bora-Nazarov Systems,
Gerhard Erker, Fang Ge, Fatma Türkyilmaz, Constantin G. Daniliuc, Gerald Kehr,
Chem. Asian J. 2015.
DOI: 10.1002/asia.201500636