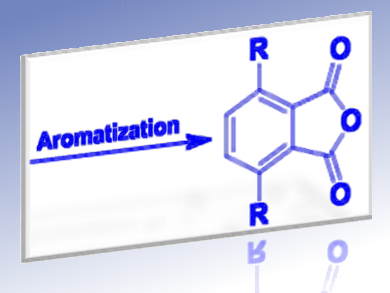
Ed de Jong, Avantium Chemicals, Amsterdam, Pieter C. Bruijnincx, Utrecht University, Daan S. van Es, Wageningen University and Research Center, all The Netherlands, and colleagues have modified the classical two-step approach to furanics-derived aromatics via, Diels–Alder (DA) aromatization, into a three-step procedure to avoid retro-DA reactions.
The new synthesis involves DA addition, followed by a mild hydrogenation step to obtain a stable oxanorbornane intermediate and to prevent any retro-DA reactivity during aromatization. This dramatically enhances the overall aromatics yield for the diene/dienophile combinations studied.
Subsequent one-pot, liquid-phase dehydration and dehydrogenation of the hydrogenated adduct using a physical mixture of two types of solid catalysts, acidic zeolites or resins in combination with metal on a carbon support (Pd/C) allows aromatization. A lactone is identified as the primary intermediate of the reaction and found to be an important precursor to all aromatic products formed in the process. Yields are as high as 84 % of total aromatics under relatively mild conditions.
According to the researchers, the new route is more generally applicable and can serve as a solution for other DA-aromatization reactions limited by the thermal stability of the intermediate adduct.
- Substituted Phthalic Anhydrides from Biobased Furanics: A New Approach to Renewable Aromatics,
Shanmugam Thiyagarajan, Homer C. Genuino, Michał Sliwa, Jan C. van der Waal, Ed de Jong, Jacco van Haveren, Bert M. Weckhuysen, Pieter C. A. Bruijnincx, Daan S. van Es,
ChemSusChem 2015.
DOI: 10.1002/cssc.201500511
Also of Interest
- CatchBio – Speaking With Consortium Partners,
ChemViews Mag. 2012.
DOI: 10.1002/chemv.201200025
B. Weckhuysen, E. Bouwnan, E. de Jong, consortium partners in CatchBio, talk about their experiences, their expectations, and why CatchBio is so unique



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)