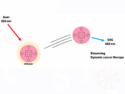
Red pyrotechnic flames are of importance for highway flares, marine distress signals, tactical signaling and for firework displays. Hitherto it was believed that highly saturated deep red pyrotechnic flames based on strontium require the presence of chlorine compounds to generate the transient strontium(I) chloride, which is a strong emitter in the red spectral range. The jointly formed strontium(I) hydroxide, always seen in the spectra of chlorinated formulations, was believed to be a vastly inferior emitter.
Ernst-Christian Koch, Technische Universität Kaiserslautern, Germany, Jesse J. Sabatini, U.S. Army Research Laboratory, (RDECOM), Maryland, USA, and colleagues found that strontium-based red pyrotechnic flares having high saturation and long dominant wavelength can be formulated entirely without chlorine compounds and thus avoid any risk for formation of suspected carcinogenic chlorocarbon compounds upon combustion.
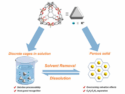
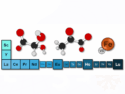
The team found that highly saturated red flames based on magnesium/strontium nitrate and a Laminac/Epon binder system can be obtained when moderate amounts of either 5-amino-1-H-tetrazole (CH3N5) or hexamine (C6H12N4) (both established pyrotechnics components) are added. They reason that the long dominant wavelength and good saturation obtained is due to strong emission of gaseous SrOH and the significant reduction of the detrimental orange emitter SrO(g). This is supported by both UV-VIS spectra and chemical equilibrium calculations. Additionally, the concomitant release of nitrogen expands the combustion plumes and helps to obtain high luminosities.
The new formulations are useful for civilian and military red burning pyrotechnics, chemically stable and are cheaper than polyvinyl chloride powder (PVC)-based formulations when hexamine is used as an additive (18 EUR/kg vs 90 EUR/kg). The use of established components rules out any unpleasant surprises with respect to chemical stability and toxicology.
- Chlorine-Free Red Burning Pyrotechnics,
J. J. Sabatini, E.-C. Koch, J. C. Poret, J. D. Moretti, S. M. Harbol,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201505829