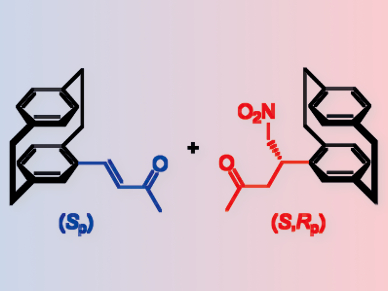
Cyclophanes are representative frameworks for planar chirality. Synthetic planar chiral cyclophanes are, for example, [2.2]paracyclophane derivatives (pictured). They are of great interest as asymmetric catalysts, and in medicinal and polymer chemistry. Producing non-racemic planar-chiral [2.2]paracyclophanes in a catalytic manner is still a challenge.
Kazuaki Kudo and colleagues, University of Tokyo, Japan, have developed a resin-supported peptide catalyst for kinetic resolution of a planar-chiral [2.2]paracyclophane derivative. This was done by a Michael addition of nitromethane to an enone substituent on the paracyclophane structure.
The resin-supported catalyst consists of a repetitive 310-helical unit, the amino acid sequence Leu-Leu-Aib, and two N-terminal tryptophan residues. The helical part of the peptide plays a critical role for the kinetic resolution. By optimizing the peptide sequence at the N-terminus of the helical chain, highly enantioselective resolution was obtained.
- Kinetic Resolution of a Planar-Chiral [2.2]Paracyclophane Derivative by Helical-Peptide-Catalyzed Michael Addition of Nitromethane,
Kengo Akagawa, Nobuhiro Nishi, Isao Yoshikawa, Kazuaki Kudo,
Eur. J. Org. Chem. 2015.
DOI: 10.1002/ejoc.201500594