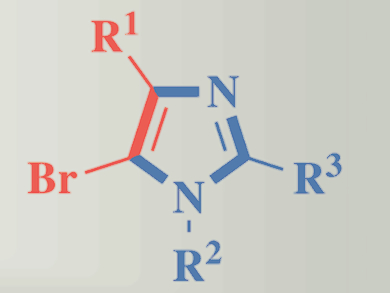
General and efficient methods for the synthesis of complex molecular skeletons are of great interest to modern organic chemistry. Multisubstituted haloheterocycles, such as haloimidazole derivatives, are versatile building blocks. Their halogen atom provides an opportunity for further functionalization through transition metal-catalyzed reactions that can form a variety of carbon-carbon and carbon-heteroatom bonds.
Yibiao Li and colleagues, Wuyi University, Jiangmen, China, have developed an annulation reaction synthesis of halogenated imidazoles from 1,1-dibromoalkenes and amidines. The copper-catalyzed cycloamination affords a diverse set of polysubstituted haloimidazole derivatives. Using 4,7-diphenyl-1,10-phenanthroline as ligand, high regio- and chemoselectivity has been achieved.
The starting materials are readily accessible and the catalytic system is of low cost. This makes this mild and general method a promising approach for the construction of these multisubstituted haloheterocycles.
- Practical Synthesis of Polysubstituted Haloimidazoles from 1,1-Dibromoalkenes and Amidines,
Yibiao Li, Liang Cheng, Yan Shao, Shaohua Jiang, Jialing Cai, Ning Qing,
Eur. J. Org. Chem. 2015.
DOI: 10.1002/ejoc.201500305