Activation of dihydrogen is a key step in catalytic hydrogenation. H2 heterolysis has been achieved using frustrated Lewis pairs and a variety of palladium salts. Silver hydrides are thought to be intermediates in Ag-catalyzed aldehyde hydrogenation reactions and have also been shown to undergo photo-induced H2 release, indicating their potential use as catalysts for H2 release from storage media such as formic acid.
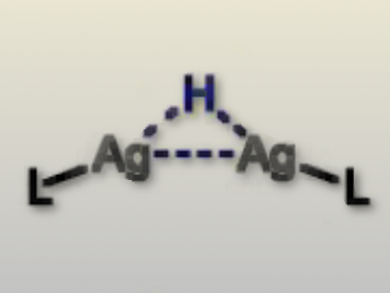
Joseph P. Sadighi and co-workers, Georgia Institute of Technology, Atlanta, GA, USA, show that silver fluorides and alkoxides undergo hydrogenolysis to form hydride-bridged disilver cations (pictured). The disilver cations form from silver alkoxides via loss of free alcohol and from silver fluorides via the generation of bifluoride as a counterion.
Kinetic isotope experiments are consistent with the deprotonation of silver-coordinated dihydrogen as the rate-limiting step. These findings represent a key step in finding new catalysts for H2 generation.
- Heterolysis of Dihydrogen by Silver Alkoxides and Fluorides,
Brandon K. Tate, Jenna T. Nguyen, John Bacsa, Joseph P. Sadighi,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500870