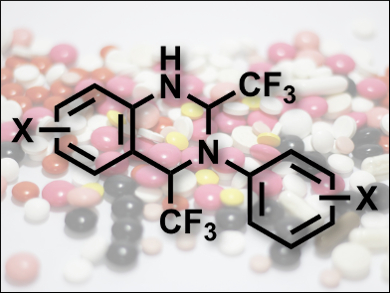
Many compounds featuring a quinazoline framework are used as therapeutic agents. Additionally, fluoro groups can improve the therapeutic activity of such compounds. While there are many synthetic protocols for the synthesis of such heterocycles, they usually involve more than one step and can have problems such as low selectivity, yield, and functional-group tolerance. Also, fluorination of the products often requires costly reagents.
G. K. Surya Prakash and Thomas Mathew, University of Southern California, Los Angeles, USA, and colleagues have developed a convenient synthesis of di- and trifluoromethyl-1,2,3,4-tetrahydroquinazolines (example pictured) which avoids these problems. They used easily available substituted anilines and di- or trifluoroacetaldehyde hemiacetals as starting materials and Lewis acids such as Ga(OTf)3 as catalysts. Using this approach, the product formed in good yields in a one-pot condensation-cyclization cascade reaction.
The researchers hope that this method will be useful for the discovery of effective fluorinated pharmaceuticals.
- Lewis Acid Catalyzed Condensation-Cyclization Cascade: Direct Synthesis of Di/Trifluoromethyl-1,2,3,4-tetrahydroquinazolines,
G. K. Surya Prakash, Attila Papp, Socrates B. Munoz, Nathan May, John-Paul Jones, Ralf Haiges, Pierre Mothé Esteves, Thomas Mathew,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500744