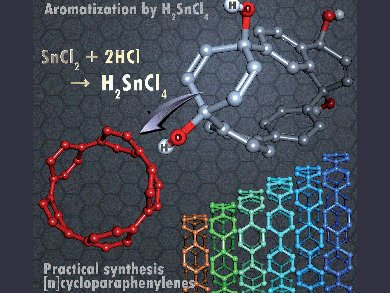
Curved π-conjugated molecules exhibit unique physical properties derived from their distorted π-orbitals. One such class of molecule, [n]cycloparaphenylenes ([n]CPPs), consists of rings of para-connected benzene rings. They display size-dependent photophysical, redox, and host–guest properties. The first synthesis of CPPs occurred in 2008, but effective methods for their mass production for potential application in molecular electronics remain limited, owing to the need for harsh conditions.
Shigeru Yamago and co-workers, Kyoto University, Japan, have developed a strategy to synthesize [n]CPPs (n = 5, 7–12) under mild conditions with yields of 56–87 %, in which the key step, reductive aromatization of a 1,4-dihydroxy-2,5-cyclohexadien-1,4-yl unit, is effected by ate complex H2SnCl4. The reaction is successfully scaled up to produce highly strained [5]CPPs on >0.3 g scale.
The prior use of H2SnCl4 in reductive aromatization reactions indicates that this strategy can be extended to enable rational design of functionalized novel π-conjugated molecules.
- Practical Synthesis of [n]Cycloparaphenylenes (n=5, 7-12) by H2SnCl4-Mediated Aromatization of 1,4-Dihydroxycyclo-2,5-diene Precursors,
Vijay Kumar Patel, Eiichi Kayahara, Shigeru Yamago,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201406650