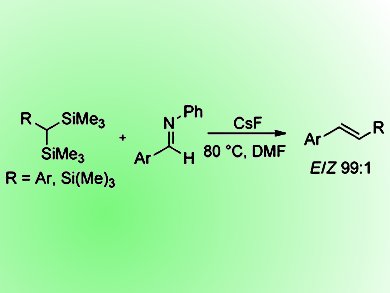
Peterson olefination, considered as a silicon analogue of the Wittig reaction, remains one of the lesser utilized methods for the conversion of carbonyls to alkenes due to two main disadvantages. The first is that the reaction currently lacks stereocontrol making it a less attractive approach for such reactions. The second is that the silyl anion generated in the reaction is derived often by the use of strong bases under nontrivial conditions prior to the addition of the aldehyde. However, if solutions to these issues could be found, the Peterson olefination would become an attractive option for these reactions as a result of its superior atom-economy over the Wittig reaction, producing low molecular silicon byproduct in the carbon—carbon double-bond-forming step rather than crystalline triphenylphosphine oxide.
Donal F. O’Shea and co-workers, Royal College of Surgeons in Ireland, Dublin, have addressed these issues by the development of bench-stable Peterson olefination reagents α,α-bis(trimethylsilyl)toluenes and tris(trimethylsilyl)methane by a general two-step synthesis. These reagents were then subsequently used for mild and E-selective olefination reactions when substituted N-benzylideneanilines were employed as electrophiles. Identification of the reaction byproduct as aqueous extractable N,N-bis(trimethylsilyl)aniline maintains the advantage of Peterson olefinations in generating a readily removable byproduct. As the use of imine electrophiles for aza-Peterson olefinations has not been previously studied, the scope of this approach is currently being further explored by the group.
- Stereoselective Peterson Olefinations from Bench-Stable Reagents and N-Phenyl Imines,
Manas Das, Atul Manvar, Maïwenn Jacolot, Marco Blangetti, Roderick Jones, Donal F. O’Shea
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500475