Phenols have gained interest because of their broad range of biological activities, wide presence in natural products, frequent utilization as intermediates and ligands in organic synthesis, and versatile applications in agrochemicals and functional materials.
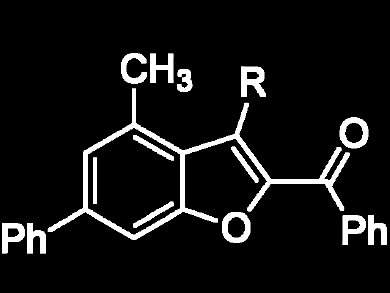
Xuesen Fan, Xinying Zhang, and colleagues, Henan Normal University, China, have developed a selective and efficient synthesis of functionalized phenols including 2-hydroxyaryl ketones through cascade reactions of 1,2-allenic ketones with commercially available β-diketones under mild conditions. The synthetic procedure is easy to handle and compatible with a variety of functional groups. The reactions were also combined with a subsequent condensation of the in-situ-formed o-hydroxyaryl ketones with an α-bromoketone to afford 2-benzoyl benzofurans.
Compared with literature methods for the preparation of substituted phenols, o-hydroxyaryl ketones, and benzofuran derivatives, the synthetic strategies developed herein have advantages such as readily available starting materials, a wide substrate scope, a simple synthetic procedure, mild reaction conditions, and high yield and selectivity.
- Synthesis of Functionalized Phenols via the Cascade Reactions of Allenic Ketones with β-Diketones,
Xuesen Fan, Meng Yan, Yan He, Nana Shen, Xinying Zhang,
Asian J. Org. Chem. 2015.
DOI: 10.1002/ajoc.201500001