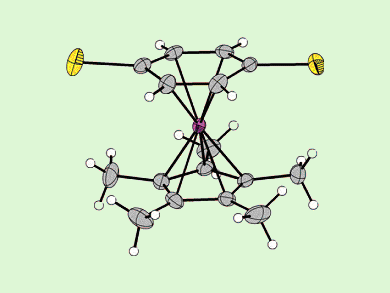
Amouri and co-workers have described the synthesis, isolation, and characterization of a new η4-diseleno-p-benzoquinone compound that is stabilized by coordination to an Cp*Ir moiety.
This organoselenium complex, [Cp*Ir(η4-C6H4Se2)], was prepared by using a template already coordinated to the Ir fragment, and thereby giving a molecule that has been elusive for several years. NMR spectroscopy and X-ray analysis data support stabilization of this reactive diselenobenzoquinone species by metal-to-ligand π backbonding.
They have also shown that this organoselenium compound has notable biological activity compared to the related quinone and thioquinone complexes, and is on a par with the cytotoxic activity of cisplatin—a promising starting point for further study.
- Discovery, Structure, and Anticancer Activity of an Iridium Complex of Diselenobenzoquinone
H. Amouri, J. Moussa, A. K. Renfrew, P. J. Dyson, M. N. Rager, L.-M. Chamoreau,
Angew. Chem. Int. Ed. 2010, 49.
DOI: 10.1002/anie.201002532 - H. Amouri, J. Moussa, A. K. Renfrew, P. J. Dyson, M. N. Rager, L.-M. Chamoreau,
Angew. Chem. 2010, 122.
DOI: 10.1002/ange.201002532