The [2+2] photocycloaddition of olefins is a well‐known model photoreaction. The [2+2] photocycloaddition of acenaphthylene (pictured below), for example, has been well known. However, the enantioselective photodimerization of its 1‐substituted derivatives had not been explored so far. Catalytic enantioselective [2+2] photocycloaddition reactions have potential applications in, e.g., natural product synthesis and drug discovery, which makes them interesting research targets.
Cheng-Yong Su, Sun Yat-Sen University, Guangzhou, China, and colleagues have developed a cage-confined catalysis protocol using chiral, photoactive metal–organic cages to promote enantioselective 2+2 photocycloadditions of acenaphthylene derivatives. The nanospace within the cage acts as a supramolecular reactor and provides both chirality and photosensitization.
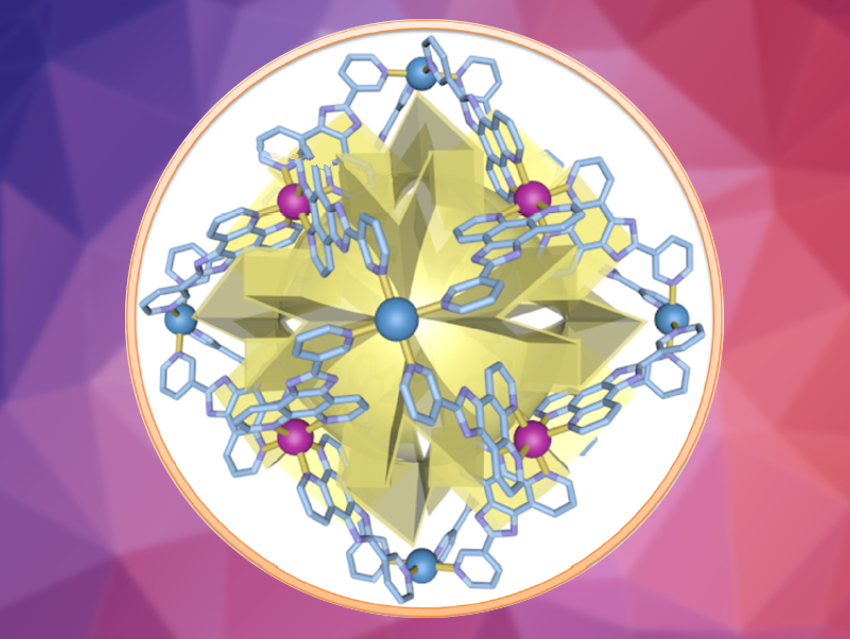
The team prepared an enantiomerically pure Δ/Λ‐[Pd6(RuL3)8]28+ metal–organic cage (Δ/Λ‐MOC‐16, pictured above, L = 2-(pyridin-3-yl)-1H-imidazo[4,5-f][1,10]phenanthroline). This cage was then used to catalyze the photocycloaddition of acenaphthylene (as a symmetrical substrate) and 1-Br-acenaphthylene (as an unsymmetrical substrate).  The reactions were performed in aqueous media and with visible light from a blue LED.
The reactions were performed in aqueous media and with visible light from a blue LED.
The team found that the cage can catalyze the desired photoadditions in a regio‐, stereo‐, and enantioselective manner. The team attributes the enantioselectivity to a preorganization of the substrates in the chiral space inside the cage, which leads to the preferential formation of one enantiomer.
- Visible-Light Photocatalysis of Asymmetric [2+2] Cycloaddition in Cage-Confined Nanospace Merging Chirality with Triplet-State Photosensitization,
Jing Guo, Yan-Zhong Fan, Yu-Lin Lu, Shao-Ping Zheng, Cheng-Yong Su,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201916722