Linear or Branched: New Silicon Peptide Biopolymers
Copolymers made from synthetic and biomimetic components open new and interesting perspectives as biocompatible, biodegradable materials that can also be given biological functionality. In the journal Angewandte Chemie, French scientists have introduced silicon-peptide copolymers. Their extremely simple synthesis is applicable to all types of peptides and produces both linear and branched polymer chains.
Silicones were a logical choice for the synthetic component of the copolymer, because they have long been used in biomedical applications, for example in parts for medical devices, catheters, and implants, as well as injectable fluids for soft-tissue augmentation. Researchers working with Ahmad Mehdi and Gilles Subra at the University of Montpellier combined these silicones with peptides as their chosen biological component.
Straightforward Polymerization
For this synthetic process, the peptides are equipped with chlorodimethylsilyl (–SiMe2Cl) groups. In the presence of water, the chloride is forced off of the silicon atom by an OH group. Pairs of Si–OH groups then react, splitting off water and forming Si–O–Si units (siloxane groups) that link together the peptide building blocks. Silicones are also held together by siloxane groups.
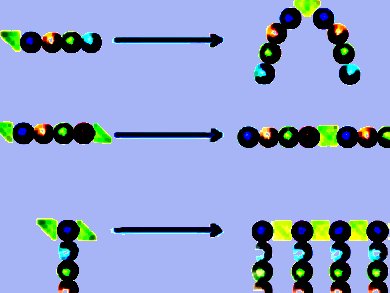
If the peptides have silicon-containing groups at only one end, the polymerization results in peptide dimers connected by a siloxane group. The dimerization of peptide ligands is a well-known strategy for improving binding affinity for various hormones and pharmaceuticals by increasing the local concentration of binders in the neighborhood of a binding site. This new dimerization technique is simpler than conventional methods, primarily because it is selective and doesn’t react with any peptide side-chains, which eliminates the need to protect them.
.jpg)
If the peptides have reactive chlorosilyl groups at both ends, the polymerization reaction forms long, linear chains of peptide and silicone units. The researchers also tested a variation in which they used peptides with lysine groups at the ends. This amino acid allows two chlorosilyl groups to be bound to the end of the peptide. The polymerization then results in a “comb” polymer with a silicone backbone that has peptide side-chains dangling from it.
The one-step polymerization reaction can be carried out in water at neutral pH and requires no catalyst or reagent. It is therefore well suited for use on an industrial scale. In principle, any desired peptide sequence can be used. By varying the building blocks and mixing different hybrid blocks in different ratios a broad palette of tailored, multifunctional biopolymers becomes accessible.
Potential applications for these hybrid polymers include systems for delivering drugs or genes into cells, antibacterial films, and the construction of bioactive scaffolds for tissue culture.
- A New Way to Silicone Based Peptide Polymers,
Said Jebors, Jeremie Ciccione, Soultan Al-Halifa, Benjamin Nottelet, Christine Enjalbal, Céline M’Kadmi, Muriel Amblard,
Prof. Ahmad Mehdi, Jean Martinez, Gilles Subra,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201411065