Halogen bonding is a non-covalent interaction featuring halogen atoms as electron acceptors. It can be compared to hydrogen bonding. The halogen atom X is attached to an electron-withdrawing group A and accepts electron density from another molecule D, forming a complex A—X- – -D.
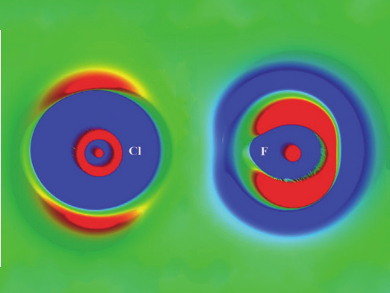
In contrast to hydrogen bonds, halogen bonds are directional, usually showing angles of 180°. This is caused by the anisotropic charge distribution at the halogen atom (see left in the picture above). The lower electron density in the direction of the A—X bond, called a σ-hole, is responsible for the halogen’s ability to act as an electron acceptor. Fluorine is different from the other halogens in that it does not typically feature a σ-hole. It can, however, form a non-covalent interaction with electron donors if the acceptor group is sufficiently electron-withdrawing.
To clarify whether these interactions can be categorized as “traditional” halogen bonds, Kiamars Eskandari and Mina Lesani, Isfahan University of Technology, Iran, have performed calculations on complexes of fluorine-containing molecules, such as F2, FCN, and C2F2, with ammonia as the electron donor. They compared these complexes to analogous chlorine and bromine compounds.
The study shows that, unlike other halogens, fluorine has a spherical charge distribution in A—F complexes (see right in the picture above), and the complexes have F- – -N bond distances greater than the sum of van der Waals-radii. Using QTAIM calculations (Quantum Theory of Atoms in Molecules), the researchers show that the majority of fluorine-nitrogen interactions are electronically quite distinct from traditional halogen bonds. The team proposes categorizing the interactions as distinct “fluorine bonds” rather than “halogen bonds” due to these differences.
- Does Fluorine Participate in Halogen Bonding?,
Kiamars Eskandari, Mina Lesani,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201405054