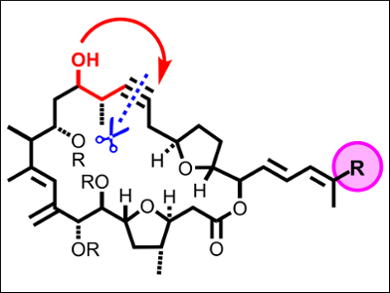
Amphidinolides (pictured) are a group of marine natural products isolated from dinoflagellates of the genus Amphidinium. Amphidinolide C and F differ in their side chains but share a common macrolide core with a signature 1,4-diketone substructure. The attraction of these targets is amplified by the dense decoration of the carbon skeleton with 12 and 11 stereocenters, respectively, two trans-configured THF-rings, and two 1,3-diene units, one of which is in a “non-thermodynamic” exo/endo-orientation. Despite the high degree of structural homology between the two amphidinolides, they exhibit strikingly different biological activities. The amphidinolide C is the most cytotoxic with IC50 values in the single-digit ng/mL range.
Alois Fürstner and colleagues, Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany, implemented a strategy for the synthesis of amphidinolide C and F using ring-closing alkyne metathesis (RCAM). While this reaction is currently gaining momentum, applications in the synthesis of complex natural products are scarce. The key intermediate in the synthesis was assembled from three building blocks by Yamaguchi esterification and Stille cross-coupling, with ring-closing alkyne metathesis (RCAM) used for the macrocyclization step.
This approach illustrates the exquisite alkynophilicity of the catalysts chosen for the RCAM and alkyne hydroalkoxylation steps, which activate triple bonds with remarkable ease but left up to five other π-systems in the respective substrates intact.
- Concise Total Syntheses of Amphidinolides C and F,
Gaëlle Valot, Damien Mailhol, Christopher S. Regens, Daniel P. O’Malley, Edouard Godineau, Hiroshi Takikawa, Petra Philipps, Alois Fürstner,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201405790


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
