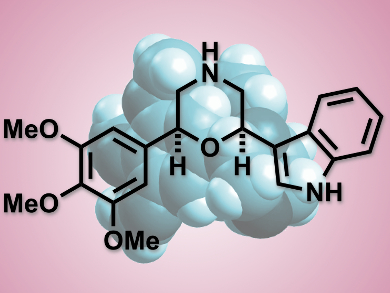
Substituted six-membered 1,4-heterocycles have attracted considerable attention because of their ubiquity in natural products and drug molecules. Morpholine is present in antimicrobial, anti-inflammatory and anti-Alzheimer compunds, and the 1,4-oxathiane scaffold is often found in biologically active molecules.
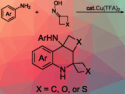
Santosh J. Gharpure, Indian Institute of Technology Bombay, Mumbai, India, and colleagues have stereoselectively synthesized cis-2,6-disubstituted morpholines and 1,4-oxathianes from readily available 1,5-diketones in a simple and efficient, Lewis acid catalyzed reductive etherification. Both symmetrical and asymmetrical derivatives could be prepared in high yields and excellent diastereoselectivities.

According to the researchers, this method could be used for the construction of cyclic sulfoxide- or sulfone-containing 1,4-heterocycles.
- Stereoselective Synthesis of cis-2,6-Disubstituted Morpholines and 1,4-Oxathianes by Intramolecular Reductive Etherification of 1,5-Diketones,
Santosh J. Gharpure, Dandela Anuradha, Jonnalagadda V. K. Prasad, Pidugu Srinivasa Rao,
Eur. J. Org. Chem. 2014.
DOI: 10.1002/ejoc.201403294