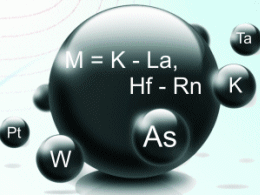
A DFT analysis of 54 cationic methylene complexes MCH2+ (M = K–La, Hf–Rn) by X. Zhang and H. Schwarz from the Technische Universität Berlin, Berlin, Germany, reveals different bonding trends for s-, p-, and d-block elements.
Also, the bonding character of the MCH2+ complexes is revealed by geometrical and molecular-orbital analysis.
MCH2+ complexes have been implicated as crucial intermediates in numerous important catalytic reactions, and understanding of both the bonding and the reactivity of naked MCH2+ sheds light on the mechanistic aspects of catalytic processes in which these simple complexes are involved.
Comparison of the periodic trends within the s-, p-, and d-block MCH2+ carbenes shows a pattern that is different for main group versus transition-metal complexes. By combining this work with the recently reported trends for the f-block lanthanide carbenes, a systematic and comprehensive overview can be obtained.

- Bonding in Cationic MCH2+ (M=K–La, Hf–Rn): A Theoretical Study on Periodic Trends
X. Zhang, H. Schwarz
Chem. Eur. J.2010, 16 (20).