Many separation techniques have been developed for the production of pure enantiomers from racemic chiral compound-forming substances. On an industrial scale this separation problem is solved mostly by classical resolution via diastereomeric salt formation.
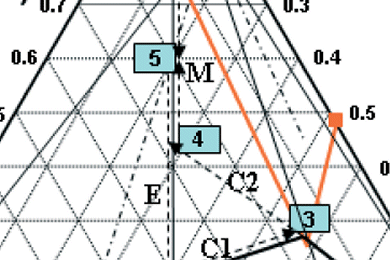
In their recent work, Andreas Seidel-Morgenstern and colleagues, Max Planck Institute for Dynamics of Complex Technical Systems, Magdeburg, Germany, investigate the separation of DL-serine via diastereomeric salt formation. For separation of DL-serine, the resolving agent 2,3-dibenzoyl-D-tartaric acid was proposed. The binary melting point phase diagram and the ternary solubility phase diagrams determined are shown and discussed together with the results. The application of the resolving agent 2,3-dibenzoyl-D-tartaric acid for the resolution of DL-serine was successful as both diastereomeric salts show perfect crystalline behavior.
- Application of Classical Resolution for Separation of DL-Serine
V. S. Sistla, J. von Langermann, H. Lorenz, A. Seidel-Morgenstern
Chem. Eng. Technol. 2010, 33 (5), 780.