The production of a cup of espresso would appear to be a simple three-step process: green coffee beans are heated dry (roasting), then ground to a fine consistency, and finally extracted under pressure with hot water.
The basic approach is repeated over 50 million times each day, but unfortunately not always with optimal results. This should really be no surprise since the metamorphosis of a mere 50 coffee beans into a cup of espresso is the purest chemistry – and no culinary masterpiece can be achieved without a basic knowledge of chemistry.
The term espresso is derived from the Italian for the verb “express” in the sense of “to force a liquid out of something by squeezing or pressing”. In other words, a distinction is being made from all other forms of coffee preparation: pressure is used to force water near the boiling point through a layer of coffee powder.
But observe the man (or woman) operating the espresso machine: First a sieve holder is released and an old batch of coffee grounds is knocked out into a special drawer. Next, precisely 6.5 g of finely ground coffee powder is introduced into the sieve holder and then pressed firmly so as to achieve a uniform distribution. Now the sieve holder is clamped in place, the pressure valve is opened, and extraction begins. After a few seconds the first aromatic espresso flows into a prewarmed cup, and within about 30 seconds the process is complete.
We begin by considering the starting material [1]: red berries from the two coffee shrubs Coffea arabica and Coffea canephora var. Robusta. Each berry is roughly 1.5 cm in diameter, and contains two yellow-green beans. Average chemical compositions for green and roasted coffee beans are provided in Tab. 1 [2].

Step 1. Roasting – Critical With Respect to Both Aroma and Flavor
In the first reaction step, green, astringent-tasting raw coffee beans are transformed into the familiar brown, aromatic beans. Up to a temperature of 150 °C the beans simply lose water; true roasting begins only above 160 °C. Chemical reactions — incalculable in number — then take place, and the constitution of the beans changes. The principal product is in fact carbon dioxide. For every kilogram of beans, as much as 12 L of CO2 will be released!
Since, during the roasting process, the very thick cell walls of the beans remain intact, released CO2 causes pressure within the cells to increase to as much as 25 bar. In other words, the chemical roasting reactions take place between 160 and 240 °C in tens of thousands of mini-autoclaves. It should come as no surprise that, under these harsh conditions, thousands of new compounds are produced in the course of thermal decomposition of the over 700 so far identified components of green coffee beans, as well as of the many polymeric storage and skeletal components [3]. From a chemical standpoint, coffee is actually the most complex beverage we consume.

Figure 1 shows compounds that have reacted during the roasting process. The most reactive are free amino acids and simple sugars like glucose, galactose, and arabinose, as well as the disaccharide saccharose (cane sugar). With increasing temperature, trigonelline 2 and the chlorogenic acids 4 are largely decomposed as well, whereas lipids and caffeine are nearly unaffected by roasting.
Chlorogenic acids are esters comprised of quinic acid 3 as the alcohol part and a p-substituted p-hydroxycinnamic acid as the acidic component. The principal representative is chlorogenic acid itself, 4. The name chlorogenic acid stems from a green color observed in the course of its alkaline oxidation, a reaction discovered in the 19th Century.
The various brown to black pigments arise through a confusing reaction cascade [4], still not clarified in detail, in which simple sugars like glucose and arabinose, for example, form caramel-like products that can in turn react further with chlorogenic acids to give red to brownish-black humic acids. Parallel to this, free amino acids react with the saccharides by way of Mailard reactions [5] to yield yellow to brownish-black melanoidins. Overall, pigment formation involves substances in every compound class, with the exception of caffeine and the fats.
The roasting process plays a decisive role with respect to both aroma and flavour. Although espresso could in principle be prepared from any coffee roast, the more darkly roasted beans are preferred, in which components have undergone more complete thermal decomposition. As a consequence, the proportion of astringent-tasting chlorogenic acids 4 is decreased [6], which explains the softer taste of espresso relative to less strongly roasted coffees. Also, trigonelline 2 is heavily decomposed, producing a multitude of heterocyclic compounds, which in turn contribute to the powerful roasted aroma. Worth noting is development in the process of the vitamin nicotinic acid (niacin) 5. Drinking a cup of espresso actually supplies roughly 15 % of the recommended daily dose of this vitamin!
Step 2: Grinding – Increases the Surface Area Available for Extraction
After roasting, the interior of the coffee beans is full of carbon dioxide as a result of decarboxylation reactions. This serves as a protective gas, preventing undesirable oxidation of aromatic components. Only over the course of several weeks after roasting is the CO2 displaced by air, allowing oxygen to pursue its oxidative mischief: the coffee ages and becomes musty. Grinding releases the protective carbon dioxide, permitting oxidation reactions to begin immediately. Thus, a good cup of espresso can only be prepared from freshly ground coffee.
Mechanical grinding of coffee serves to greatly increase its surface area, which in turn facilitates the extraction process. The coffee also becomes heated during grinding, however, and poor milling practices allow temperatures as high as 100 °C to be attained. A good coffee mill is so constructed that the warming phase persists for only a few seconds, and the rise in temperature of the mill’s grist is minimized. The coffee mill is in fact just as important for the quality of espresso as the espresso machine itself. In home brewing this is the place that most often suffers from false economising.
Coffee powder for the preparation of espresso should have a particle size in the range 0.3 – 0.4 mm (ordinary filter coffee: 0.4 – 0.6 mm), although the goal is not to achieve homogeneity. On the contrary: only a broad distribution of particle sizes guarantees an optimal throughput time for the hot, pressurized water.
Step 3: Extraction – of the Most Desirable Components, from a Sensory Standpoint
Passage of a solvent (hot water) through a solid phase (coffee powder) under pressure is very simple from an apparatus perspective, and is reminiscent in some ways of high-pressure liquid chromatography (HPLC). For laminar flow of a solvent through a cylindrical column (radius r, length L) filled with porous particles (diameter d), Darcy’s law permits derivation of the following expression for approximating the relationship between pressure difference and volume velocity V/t [7, 8]:

 Such boundary conditions as amount of coffee, water temperature, diameter of the filter, applied pressure, and extraction time have been optimized empirically in thousands of Italian espresso bars over decades.
Such boundary conditions as amount of coffee, water temperature, diameter of the filter, applied pressure, and extraction time have been optimized empirically in thousands of Italian espresso bars over decades.
Current established standards are:
- Filter radius 3.5 cm,
- Quantity of water 30 mL,
- Coffee powder 6.5 ± 1.5 g,
- Pressure 9 ± 2 bar,
- Water temperature 90 ± 5 °C.
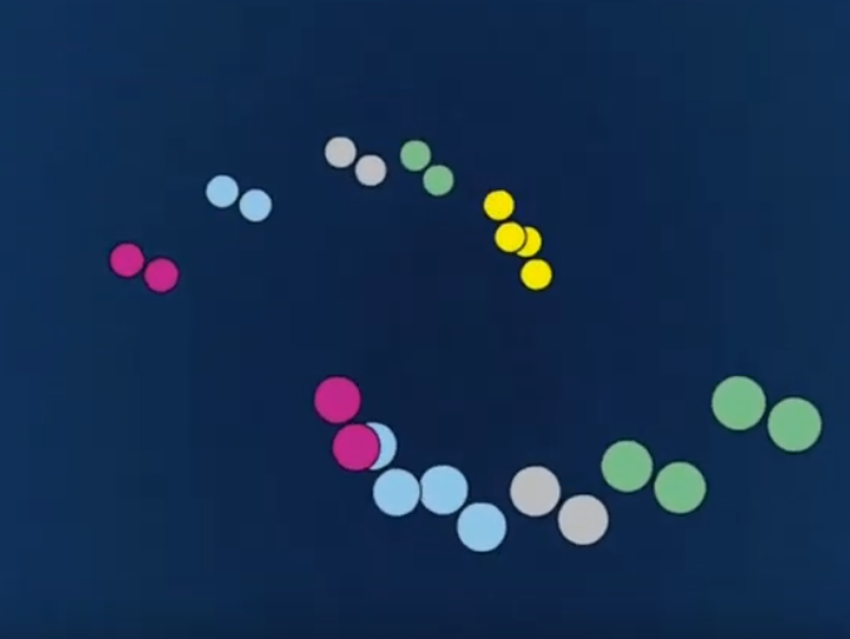
Prior to extraction the coffee powder is dry, so it takes several seconds before the first espresso trickles into the cup. After that, one would expect a constant volume velocity to be established, which, with the proper particle size and machine dimensions, would lead to the desired cupful of espresso in 30 ± 5 s. Figure 2 displays a set of actual experimental findings.
Somewhat sheepishly, it must be acknowledged that preparation of a cup of espresso is evidently much more complicated than a typical high-pressure chromatography run, since the theoretical predictions don’t correspond at all with practice.

So where is the error in reasoning? The following brilliant experiment, devised by Baldini and Petracco [9], sets us on the right track:
The extraction process is interrupted after 12 s, the filter cake is inverted through 180°, after which extraction is resumed. Following this step, which amounts to a reversal of the flow direction, an identical flow profile is registered (see Fig. 2). It follows that neither the extraction itself nor swelling of the coffee powder is responsible for the observed phenomenon, but rather some sort of time-dependent hydraulic resistance.
A glance through a microscope reveals that grist from the coffee mill is not homogeneous (see Fig. 3). Under the applied pressure, as a water front moves it carries with it the smallest coffee particles, which then travel past the larger ones to congregate at the bottom of the layer of coffee powder. The resulting partial blockage leads to an increase in hydraulic resistance, and the flow velocity decreases (see Figs. 2a, b). In the experiment described, if the flow direction is now reversed, small particles again move in the flow direction (see Fig. 2c). At first the hydraulic pressure decreases, because the “blockage” disappears (see Fig. 2d) until small particles once again collect — at the other end — and hydraulic resistance increases once more (see Fig. 2e).

But the chemical processes occurring in an espresso machine are even more complex. During the brief extraction period, equilibrium cannot be established between the phases, and only 75 % of the highly soluble caffeine is extracted. This incomplete extraction would at first appear to be a shortcoming, but in fact perfection lies in this defect: many components with undesirable sensory effects are left behind, as a result of which espresso is more readily digestible than ordinary brewed filter coffee.
It is not just water-soluble compounds that are extracted; the hot water also causes the melting of lipids that have diffused to the surface after roasting, and the rapid streaming between coffee particles leads to formation of a fine lipid emulsion, with drop sizes between 0.5 and 1.0 µm. Dissolved in these fat droplets are aromatic substances that would otherwise evaporate upon departure of the hot liquid.
But there is no need to worry. The fat content of espresso is very low, and even those obsessed with such things have absolutely no reason to suffer a guilty conscience over a mere 9 kcal.
References
[1] H. G. Maier, Chem. unserer Zeit 1984, 18, 17. DOI: 10.1002/ciuz.19840180103
[2] A. Illy, R.Viani, Espresso Coffee: The Chemistry of Quality, Academic Press, London 1998.
[3] I. Flament, Coffee Flavor Chemistry, John Wiley & Sons, Chichester 2002.
[4] R. Viani, Ullmann’s Encyclopedia of Industrial Chemistry, Vol. A 7, Wiley-VCH, Weinheim 1996, 315–339
[5] M. Angrick, D. Rewicki, Chem. unserer Zeit 1980, 14, 149. DOI: 10.1002/ciuz.19800140503
[6] R. Viani, AU J. Technol. 2002, 6 (1), http://www.journal.au.edu/au_techno/2002/jul2002/index.html
[7] C. F. Poole, S. K. Poole, Chromatography Today, Elsevier, Amsterdam 1991.
[8] H. Engelhardt, Hochdruck-Flüssigkeits-Chromatographie, Springer, Berlin 1977.
[9] G. Baldini, M. Petracco, 7th Conf. Eur. Cons. Math. Ind. 1993, cited in [2].
The article has been published in German in Chem. Unserer Zeit 2003, 37 (3), 215–217 and was trnslated by W. Russey
In part 2 …
Espresso – A Feast For the Senses
Klaus Roth describes the thrill of anticipation commencing with a scintilating glance at the crema, the foamy consolidated surface layer of an espresso. This arises through an interplay of emulsified fats, denaturatedd proteins, and surface-active compounds.
Other articles by Klaus Roth published by ChemistryViews magazine:
- In Sparkling Wine, Champagne & Co
Klaus Roth shows that only chemistry can be this tingling
DOI: 10.1002/chemv.201000042 - In Chemistry of a Hangover — Alcohol and its Consequences
Klaus Roth asks how a tiny molecule like ethanol can be at the root of so much human misery?
DOI: 10.1002/chemv.201000074 - In Chocolate — The Noblest Polymorphism
Klaus Roth proves only chemistry is able to produce such a celestial pleasure
DOI: 10.1002/chemv.201000021 - In The Chemist’s Fear of the Fugu
Klaus Roth shows the chemist’s fear of the fugu or pufferfish extends as far as the distinctive and intriguing poision it carries
DOI: 10.1002/chemv.201000104
Also of Interest