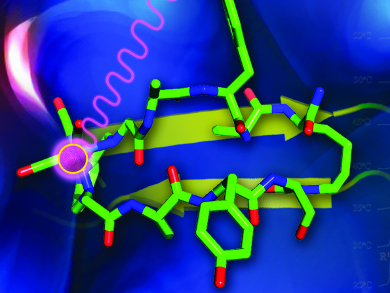
In designing peptide-based rhenium/technetium-containing diagnostic and therapeutic probes, incorporating the metal complex directly into the structure of the biomolecule is of great interest. Such an approach makes the labelling element a critical component of the biologically active region and essentially “hides” the metal core within the ligand framework. This produces a more stable, compact, lower molecular weight chelation system.
Leonard G. Luyt, University of Western Ontario, London, Canada, and his colleagues have explored such a design concept. They used cyclic peptides to act as scaffolds to emulate active β-sheet regions, which account for over 30 % of all secondary structural conformations found in proteins. The researchers included a tridentate chelation core in the peptide for the incorporation of a rhenium/technetium radiolabel. Variable-temperature 1H NMR spectroscopy was used to investigate intramolecular hydrogen bonding in the macrocyclic peptides. Computational modelling and circular dichroism spectroscopic analysis revealed that the peptide backbone exists in a similar conformation both before and after metal coordination.
Thus the seamless incorporation of a tridentate chelation core into the backbone of a macrocyclic peptide can greatly help the design of metal-centric peptidomimetic imaging agents without disrupting the secondary structure.
- An Integrated Imaging Probe Design: The Synthesis of 99mTc/Re-Containing Macrocyclic Peptide Scaffolds,
Jennifer L. Hickey, Emily J. Simpson, Jinqiang Hou, Leonard G. Luyt,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201404774