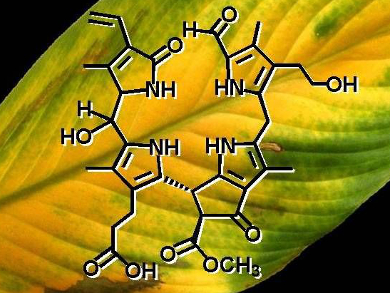
The color changes that we see in autumn as leaves change from green to yellow are a result of the breakdown of chlorophyll. It is first transformed into colorless tetrapyrroles, so-called phyllobilanes, which are then degraded further into yellow oxidation products. In extracts of a variety of yellow, senescent leaves, colorless “non-fluorescent” chlorophyll catabolites (NCCs) and “dioxobilin-type” NCCs (DNCCs) were found to accumulate. These appeared to represent the “final” stages of metabolically controlled chlorophyll breakdown.
Bernhard Kräutler and his group at the University of Innsbruck, Austria, have been investigating the selective oxidation of natural NCCs in homogenized Spathiphyllum wallisii leaves. They found that 15-hydroxy- and 15-methoxy-NCCs are formed with high stereo- and regioselectivity. Such “oxidized” NCCs are precursors for a known yellow chlorophyll catabolite (YCC), thus providing an endogenous oxidative path from NCCs to YCCs that may be active in leaves.
The researchers believe that such an oxidative path involving NCCs may open a natural route to further breakdown via colored chlorophyll catabites, and to eventual complete degradation of tetrapyrrolic phyllobilins, a pathway that until now has remained elusive.
- Stereo- and Regioselective Phyllobilane Oxidation in Leaf Homogenates of the Peace Lily (Spathiphyllum wallisii): Hypothetical Endogenous Path to Yellow Chlorophyll Catabolites,
Clemens Vergeiner, Markus Ulrich, Chengjie Li, Xiujun Liu, Thomas Müller, Bernhard Kräutler,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201404783