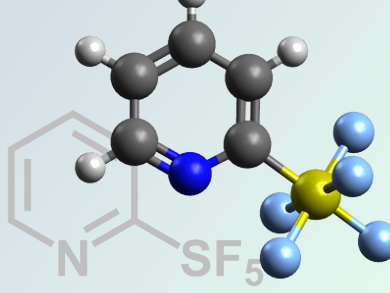
The pentafluorosulfanyl (SF5) group is important for the development of new materials, pharmaceuticals, and agrochemicals.
William R. Dolbier, Jr. and Oleksandr S. Kanishchev, University of Florida, Gainesville, USA, have synthesized a series of new substituted 2-pyridylsulfur pentafluorides, a moderately expensive and highly efficient chlorine-fluorine exchange reagent.
Excess oxidative fluorination of 2,2’-dipyridyl disulfides with a KF/Cl2/MeCN system leads to the formation of thirteen new 2-pyridylsulfur chlorotetrafluorides. These molecules were found to undergo further chlorine–fluorine exchange reactions with silver(I) fluoride. This enables ready access to a series of ten new substituted 2-pyridylsulfur pentafluorides.
This is the first preparatively simple and readily scalable example of the transformation of an existing heterocyclic sulfur functionality to prepare SF5-substituted heterocycles.
- Synthesis and Characterization of 2-Pyridylsulfur Pentafluorides,
Oleksandr S. Kanishchev, William R. Dolbier Jr.,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201409990


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
