Tomoyuki Mochida and colleagues, Kobe University, Japan, have developed solvent-recognition films that enable visual recognition of solvents without any analytical instruments.
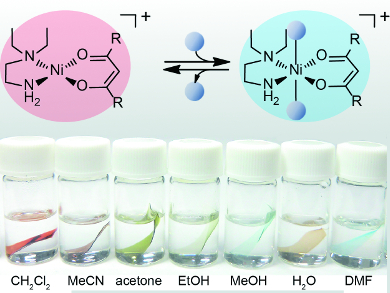
The films were prepared by incorporating solvatochromic nickel(II) complexes into Nafion membranes. Nafion is a perfluorinated ion-exchange polymer bearing nanopores approximately 40 Å in diameter and sulfonic groups on its side chain. The sulfonic protons in the polymer can be replaced with various cations, in this case, cationic nickel-chelate complexes of the type [Ni(L)(L’)]+ (HL = acetylacetone; L’ = N,N-diethylethylenediamine).
Immersion of the films in a solvent causes the transparent film to change to a color from red to pale blue green, depending on the donor number of the solvent. The color change is based on an equilibrium shift between square-planar and solvent-coordinated octahedral geometries of the cations. The films can distinguish similar solvents such as MeOH, EtOH, 1-PrOH, and 2-PrOH and are also useful for detection of the alcohol concentration in water.
The Nafion-based films exhibit a reversible solvent response. They are easily prepared, robust, and reusable and the incorporated complexes do not leach into the solvents, making them ideal for various applications in the chemical and engineering industries.
- Colorimetric Solvent Indicators Based on Nafion Membranes Incorporating Nickel(II)-Chelate Complexes,
Hitoshi Hosokawa, Yusuke Funasako, Tomoyuki Mochida,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403996