Preparation and Characterization of Carbonic Acid
Until fairly recently, scientists were convinced that carbonic acid (H2CO3) does not exist as a stable molecule. In the journal Angewandte Chemie, German researchers have now introduced a simple pyrolytic method for the production of gas-phase carbonic acid that allowed the spectroscopic characterization of gas-phase carbonic acid and its monomethyl ester.
Carbonic acid is a physiologically important molecule. For example, it helps maintain a constant pH value for blood and is an important intermediate in the formation of the carbon dioxide we exhale. It is also likely to play an essential role in CO2 sequestration technologies. There is also much evidence for the presence of solid carbonic acid in extraterrestrial ice, including on the surface of Mars, and in interstellar regions.
Synthesis by Pyrolysis
A team led by Peter R. Schreiner at the University of Gießen has now developed a novel and broadly applicable method for the production of gas-phase carbonic acid. Their technique is based on the pyrolysis of a readily available precursor molecule (di-tert-butyl dicarbonate) in the gas phase. The resulting carbonic acid is trapped in an extremely cold, noble gas matrix.
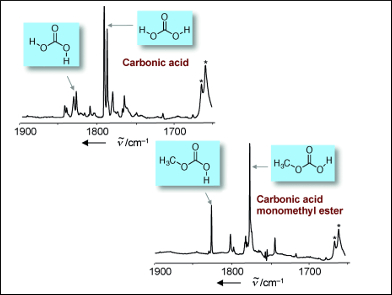
By starting with a different precursor molecule, the researchers were able to extend their new pyrolysis technique to obtain gas-phase carbonic acid monomethyl ester for the first time. Trapped in their icy matrix, the carbonic acid and carbonic acid monomethyl ester could be subjected to exhaustive infrared spectroscopic studies for the first time. Comparison of the data with theoretically calculated values showed excellent agreement.
Two Forms of Carbonic Acid?
The results of these experiments bring new insight into a matter of controversy concerning carbonic acid: are there really, as was proposed by several teams of researchers some time ago, two different crystalline forms of carbonic acid, the alpha and beta forms? Schreiner and his co-workers say this is not the case. Their spectroscopic data agree perfectly with the vapor phase above the theoretical beta form, but not the vapor phase of the solid thought to be the alpha form of carbonic acid. However, the spectra of the carbonic acid monomethyl ester correspond beautifully to this supposed alpha-carbonic acid. Says Schreiner: “It is clear that the molecule previously thought to be the alpha form of carbonic acid is actually the carbonic acid monomethyl ester.” This conclusion is in accord with the method used to prepare the “alpha form”, which uses methanol as a solvent under acidic conditions that favor an esterification reaction between carbonic acid and methanol.
“Our results shed new light on the gas-phase chemistry of carbonic acid,” says Schreiner. “They will be highly useful for the identification of carbonic acid in the environment and the atmosphere, as well as in astrophysical research.”
- Gas-Phase Preparation of Carbonic Acid and Its Monomethyl Ester,
Hans Peter Reisenauer, J. Philipp Wagner, Peter R. Schreiner,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201406969