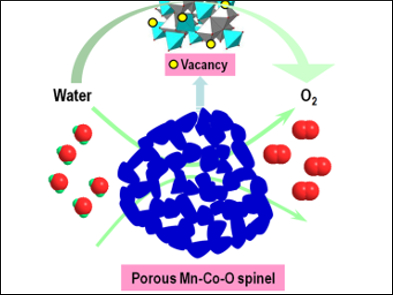
The electrochemical oxygen evolution reaction (OER) plays a key role in several energy-storage strategies, including in fuel cells, metal-air batteries, and the generation of hydrogen and oxygen through water splitting. However, to proceed efficiently, this reaction requires a durable electrocatalyst.
Shi Zhang Qiao and colleagues, University of Adelaide, Australia, have developed catalysts based on spinel Co3O4 with tunable amounts of Mn. They used a self-templating process to give the material a high surface area and ensure that the material was porous enough to allow for the transport of reactants and products during the OER. Porosity was generated by precipitating nanoscale KCl particles during the synthesis. These crystals functioned as sacrificial templates and left behind pores once the material was washed with water. Interestingly, the KCl crystals also protected defect sites during the synthesis, which increased the number of surface oxygen vacancies in the final material.
The resulting catalysts were highly active and delivered a current density of 10 mA cm–2 at 1.58 V versus the reversible hydrogen electrode in 0.1 M KOH solution, which is comparable to or better than state-of-the-art catalysts for this reaction, which are often based on expensive and scarce noble metals.
- Synthesis of Highly Active and Stable Spinel-Type Oxygen Evolution Electrocatalysts by a Rapid Inorganic Self-Templating Method,
Tian Yi Ma, Sheng Dai, Mietek Jaroniec, Shi Zhang Qiao,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403946