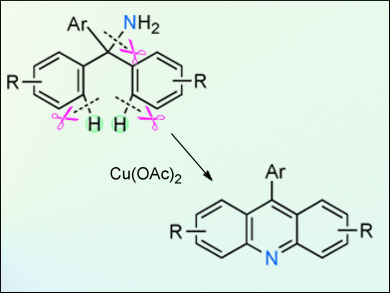
An unprecedented cyclization of tritylamines through C–H and C–N bond cleavage to produce acridine derivatives has been developed by Tetsuya Satoh, Masahiro Miura, and colleagues, Osaka University, Japan. This cyclization is promoted by copper acetate under an O2 atmosphere. The resulting 9-phenylacridine derivatives are of interest because of their biological properties and their unique optical, electro-, and photochemical properties. Some of the obtained derivatives exhibit intense fluorescence in the solid state.
Mechanistic investigations consisting of 15N-labeled crossover experiments suggest that this cyclization may involve an associative pathway with respect to the C–N bond formation after the first C–H bond-cleavage step. A range of substituted triarylmethylamines were examined and it was found that even halogenated and highly substituted substrates underwent the cyclization smoothly, although longer reaction times were sometimes required.
This unexpected cyclization offers an alternative synthetic route to a class of molecules with interesting properties.
- Unexpected Cyclization of Tritylamines Promoted by Copper Salt through C–H and C–N Bond Cleavages to Produce Acridine Derivatives,
Ryosuke Morioka, Koji Hirano, Tetsuya Satoh, Masahiro Miura,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201404656